Slow down for the win 7 Apr 5:45 PM (7 days ago)
ACRF running coach Paul Hadfield shares expert running tips to help you start training for your next race. Learn more about Paul here.
One of the best predictors of success in distance running is the ability to accumulate big weekly mileage. Now I don’t mean tipping over the 200km per week mark like the World’s best marathoner Eluid Kipchoge but the more we can build our mileage, in a safe progressive way, the more likely we are to reach our big race goals.
The best way to achieve bigger weekly distance is simple. Slow down!
While it may seem counterintuitive, slow running is our secret weapon and offers numerous benefits to our endurance engine.
Running at a slow, steady pace:
** allows the body to work within its aerobic zone
** allows you to efficiently burn fat as fuel
** increases the number of mitochondria (oxygen carrying power packs) in muscle cells. This helps improve your ability to deliver oxygen to muscles and sustain longer efforts without fatiguing.
** helps to reduce the risk of injury by minimising the impact on the body and providing an opportunity for muscles and joints to adapt to the repetition of running without excess strain.
So how slow can you go?
Kipchoge’s race pace for the marathon is 2:50/km (read that again!) but he spends 85% of his running week between 4min and 5min/km’s. I think it’s safe to say that the average punter could learn from the G.O.A.T. and drop their pace by 1 to 2mins/km from their marathon goal pace for most of the week.
This will allow us to be fresh when we do decide to crank up the speed and help us to back up week after week without ending up on the physio’s table.
If we slow things down and add to our weekly distance by 10% per week the sky’s the limit.
Happy Running!

The post Slow down for the win appeared first on ACRF.
Running is powerful, Joe’s story of motivation 7 Apr 5:14 PM (7 days ago)
The post Running is powerful, Joe’s story of motivation appeared first on ACRF.
Michael’s leaving a legacy in his will – he’s among the few who do 25 Mar 7:28 PM (20 days ago)
Around $150 billion is expected to be inherited this year, yet only one per cent of that is gifted to charities. One man explains why his will leaves a gift of kindness to strangers.
TRANSCRIPT
Michael Ow is a migrant from Malaysia who has just prepared a new will.
The 55 year-old works at the Australian Cancer Research Foundation and, with the blessing of his family, has left the charity a small bequest.
“One per cent of whatever I’ve left when passed. So hopefully that will go towards some cancer research. We discussed it with our children and they said, yeah, that’s great. Everybody needs to do their part in terms of contributing back to the community in terms of ‘right, we’ve got to cure this disease, cure cancer.'”
And there is another reason Mr Ow would like to help beat a disease that claims 60,000 lives in Australia each year.
As a teenager he lost his younger cousin Pui Fun to leukaemia.
Decades later, it’s his way to honour her memory.
“Growing up she was almost like a little sister to me. That was very heartbreaking to see that one day she’s not there any more at family gathering. I feel that leaving this gifting will not just benefit my family in terms of finding a cure, for cancer research. It will also help other families.”
It’s a gift the Australian Cancer Research Foundation is grateful to receive to help further its often groundbreaking research.
CEO Kerry Strydom explains:
“Over two in five people in Australia will be diagnosed with cancer by the time that they’re 85. That’s quite an alarming statistic. At the moment we are looking at about 165,000 people diagnosed with cancer each year. So, it’s really, really important that we conduct this research, we investigate new things, innovative ideas.”
The Productivity Commission estimates that $3.5 trillion in assets will be transferred between generations in Australia by 2050.
However only a fraction will go to good causes.
And the cost-of-living crisis is also having an impact, according to a decade of tax data.
KPMG Economist Terry Rawnsley explains.
“What we’ve seen over the last 10 years, when interest rates increase, wages are growing slowly, we see less people giving to charities. These are people who are probably having problems getting food on the table for themselves.”
It’s not just medical research that’s missing out.
The Red Cross provides vital services to millions impacted by natural disasters and social crises.
Deputy CEO Penny Harrison says bequests are badly needed.
“The extraordinary gifts of a legacy from someone passing funds through their will makes an incredible difference not only, for example, to the way that we can train and prepare volunteers but also to invest in some of those core services that are required by Australian Red Cross year round, for example support to asylum seekers and refugees.”
Like many charities, the Australian Red Cross has specialist lawyers to assist people to prepare a simple will, including a bequest.
Ms Harrison explains.
“One of our solicitor networks, they will provide a considerably reduced fee to really support you prepare your will well. And they are willing to hold one-off consultations with referred Australian Red Cross supporters because the will reflects who you are and what’s important to you, to your loved ones, family and causes and charities that are somehow meaningful for you. It’s the way in which you can gift your assets for the future.”
For Michael Ow, living well means talking about death and preparing for the inevitable.
“The process of leaving a gift in a will is quite easy. Many people think that it costs a lot of money. Many people think it’s a very complicated process and as mentioned, a lot of people think it’s taboo subject. They don’t want to talk about death, they don’t want to talk about leaving it. We need to encourage more people in our culture to provide gift in will to charities to support great causes like cancer research.”
However, he says leaving a legacy means more than just money.
“My advice to everyone is cherish those people around you. Dance as if there’s no tomorrow. Live your life today and tell them that you love them!”
Podcast transcript sourced from SBS News In Depth here.
The post Michael’s leaving a legacy in his will – he’s among the few who do appeared first on ACRF.
Amid Australia’s $3.5 trillion wealth transfer, Michael is making an unusual choice 25 Mar 7:17 PM (20 days ago)
Trillions of dollars in assets is set to be transferred between generations by 2050, but only a small portion will go towards good causes. One man who’s left a legacy to charity in his will hopes to inspire others.
Michael Ow is 55 and working full-time as a sales manager. He recently made a very important decision while finalising a new will.
“I am gifting a small percentage, around 1 per cent of whatever I have left, when I pass,” he said.
Ow works at the Australian Cancer Research Foundation (ACRF), and has chosen to leave a bequest to support cancer research.
“My wife and I discussed it with our children, and they are very happy for me to do this,” he said.
But there’s another reason for his decision to support medical research.
A ‘heartbreaking’ loss
As a teenager in a large Chinese family, Ow experienced the loss of a younger cousin to leukaemia while living in Malaysia.
“Pui Fun was like a little sister to me,” Ow said while looking at photos. “It is still very hard thinking about her now.”
He said Pui Fun passed around the age of 14.
“It was heartbreaking. One day, she just wasn’t here any more,” he recalled.
“Our parents hushed up about it because they didn’t think young people should know about death; it was a taboo subject.”
He said bequeathing would help him remember her as someone dear to him.
“And I hope that by leaving this gift, it will not just benefit my family. It will also help other families in terms of finding a cure through cancer research.”
How many people leave money to charity?
But not everyone thinks the same way.
Fewer than half of all people in Australia have a will. Of those, only 6.5 per cent make provisions for charity.
JBWere, NAB’s private wealth company, predicted that $150 billion would be inherited in 2024, and only 1 per cent of that would go to charities — including cancer research.
Australia ranks among the world’s wealthiest nations, yet the report revealed that gifts in wills lag behind those of other developed countries.
In the United States, 4.4 per cent of inherited wealth ($69 billion) is donated through bequests, while in the United Kingdom, 3.7 per cent ($7.6 billion) is donated. New Zealand bequests are on par with Australia, at about 1 per cent of all inheritances.
Australia is also entering the greatest wealth transfer in its history.
The Productivity Commission estimates $3.5 trillion in assets will change hands by 2050, as baby boomers pass wealth to younger generations.
‘Unforgettable difference for generations’
Kerry Strydom, CEO of ACRF, said: “There are more research projects that we would like to fund than we can afford.”
ACRF has so far funded 90 research projects across Australia totalling $204 million. Among those is seed funding for the development of a now widely adopted cervical cancer vaccine.
Charities, including ACRF and the Australian Red Cross (ARC), can assist people in drawing up a simplified will that includes a gift to charity.
Penny Harrison, deputy CEO of the ARC, said: “A will reflects who you are, and if causes like charities are meaningful to you, this is one way to gift assets for the future.
“A bequest can make an unforgettable difference for generations to come, both in Australia and around the world.”
However, most charitable giving has declined in recent years, according to financial services company KPMG.
Terry Rawnsley, an economist at the financial services firm, said about 30 per cent of Australian taxpayers — about 4.3 million people — make a charitable donation deduction when filing their tax returns.
“Over the past 10 years, analysis of Australian Taxation Office data shows a decline of 275,000 households giving an annual donation,” he said.
“As cost of living pressures bite, people are also making smaller donations with an average of $1,000 annually.”
But those who can afford to give are digging deeper, according to KPMG.
As Australia’s population ages, new trends are also emerging.
Rawnsley said older Australians are changing the way they hold their assets, “selling homes, getting out of the share market and putting wealth into cash”.
“This big generational transfer of wealth can actually worsen income and wealth inequality in the community,” Rawnsley said.
“Yet, charitable donations can provide enormous value, whether by helping to reduce homelessness or supporting a particular cohort that donors feel very strongly about and want to assist after they are gone.”
‘Cherish those around you’
For Ow, living well means talking about death and preparing for the inevitable.
“Some people think making a will is a taboo subject. They don’t want to talk about death or leaving their assets,” Ow said.
However, Ow said leaving a legacy can mean more than just money.
“My advice to everyone is to cherish those people around you when you have time with them.
“Dance as if there’s no tomorrow. Live your life today and tell them how much you care,” he said.
Article sourced from SBS News here.
The post Amid Australia’s $3.5 trillion wealth transfer, Michael is making an unusual choice appeared first on ACRF.
Record $3.5 million injection of hope for ovarian cancer research 20 Mar 4:56 PM (25 days ago)
The Ovarian Cancer Research Foundation (OCRF) today announced its largest-ever funding distribution with $3.5M dedicated to cutting-edge ovarian cancer research.
Eight projects have received funding from the OCRF’s 2025 National Research Grants Program. Awardees include Australian researchers who are among the few in the world with programs dedicated to rare ovarian cancer subtypes.
The OCRF grant recipients are from five institutions including the Peter MacCallum Cancer Centre, Hudson Institute of Medical Research, QUT, Griffith University and QIMR Berghofer.
Ovarian cancer is the most lethal gynaecological cancer.
Eight innovative medical research projects will share in a record $3.5 million awarded in the Ovarian Cancer Research Foundation’s 2025 National Research Grants Program.
This is the largest funding distribution in the OCRF’s 25-year history, reflecting the foundation’s growing success in fundraising and awareness, and a strong drive in the broader community to get behind the cause and change outcomes for the most lethal gynaecological cancer. Current average five-year survival rate for women diagnosed with ovarian cancer is just 49 per cent, and this drops to 29 per cent for women diagnosed at an advanced stage.
“I believe we are on the cusp of change for ovarian cancer. For too long the statistics have been stubborn and progress toward effective methods of early detection, and development of enduring successful treatments, has been too slow,” said Robin Penty, OCRF’s Chief Executive Officer.
“Momentum, however, is building. This new funding is critical, and these funded projects hold great promise. There’s still a long way to go, but, combined with strong advocacy to government and vital research collaborations in Australia, and overseas, there is reason for renewed hope in the effort to overcome this feared disease.”
Five of the eight grants are newly supported by the OCRF, including the first ever projects supported by the Mother’s Day Classic Foundation in association with the OCRF. Another is an extension of an existing OCRF grant and a further two grants are collaborations with the Australian Cancer Research Foundation.
The new 2025 Ovarian Cancer Research Foundation National Research Grant Program major grant recipients are:
Associate Professor Simon Chu, Hudson Institute of Medical Research and Monash University, $724,293 over three years: study focusing on a specific mutation present in adult granulosa cell ovarian tumours and its interaction with a family of proteins, to develop a new treatment for this rare subtype.
Associate Professor Kylie Gorringe, The University of Melbourne and Peter MacCallum Cancer Centre, OCRF Mother’s Day Classic Foundation grantee*, $481,667 over three years, to examine the proteins present in mucinous ovarian cancer to identify ways to therapeutically target the disease, and screen drugs to find new treatment approaches for this rare subtype.
Dr Emma Bolderson, QUT, $460,552 over two years to investigate whether targeting a process in DNA damage repair involving lactate could provide the foundations of an effective treatment approach for rare ovarian cancer clear-cell carcinoma.
Dr Dale Garsed, The University of Melbourne and Peter MacCallum Cancer Centre: $299,584 over three years to examine the immune response to cancer in long-term survivors of high-grade serous ovarian cancer to determine how antibody-producing immune cells promote survival, and whether this information can be leveraged to develop new treatments.
Dr Nicole Campbell, Hudson Institute of Medical Research, OCRF Mother’s Day Classic Foundation grantee*: $892,212 over three years, to study a new immunotherapy that targets high-grade serous ovarian cancer by focusing on a naturally-produced protein known as interferon epsilon, which can help activate the immune system.
Notably, three of the major grants will examine treatment options for more rare subtypes of ovarian cancer. One of the most challenging aspects of ovarian cancer is the diversity and complexity of the disease, so it’s vital to support research into the rarer types, to ensure better outcomes for everyone who receives an ovarian cancer diagnosis.
In addition to the five major grants for new projects, the OCRF will support three ongoing projects:
$473,458.00 to Professor Michael Jennings, Griffith University, investigating a promising sugar-based biomarker early detection approach (final year expansion of current grant)
Collaborative Grant to support ovarian cancer research at the Australian Cancer Research Foundation (ACRF) Centre for Optimised Cancer Therapy at QIMR Berghofer, $100,000 (one year)
Collaborative Grant to support ovarian cancer research at the ACRF Centre of Advanced Image-Guided Cancer Therapeutics at Peter MacCallum Cancer Centre, $100,000
(one year)
All this research funding is 100 per cent enabled by the Australian community, which raises every penny the OCRF awards for ovarian cancer research.
“I’m genuinely in awe of the collaborative and generous spirit at the heart of the OCRF community. From our incredible partners and corporate donors to every fundraiser and community member who puts their time, precious dollars and passion into this cause,” concluded Ms Penty. “To be awarding our largest ever grant distribution illustrates that the entire ovarian cancer community is behind the dedicated researchers working in the lab. The OCRF is proud to be entrusted with investing in the best and most promising research projects possible.”
Grant applications are rigorously assessed by the OCRF’s International Scientific Advisory Committee and Consumer Representative Panel.
These major research investments are a testament to the commitment and energy of the OCRF community, and a shared determination to change the future for generations of women and girls toward a healthy vital future for all those impacted by ovarian cancer.
*The projects led by Dr Nicole Campbell and Associate Professor Kylie Gorringe are supported Mother’s Day Classic Foundation in association with the OCRF.
Media release sourced from the Ovarian Cancer Research Foundation here.
The post Record $3.5 million injection of hope for ovarian cancer research appeared first on ACRF.
The end of cancer: how cell therapy breakthroughs have us on the edge of a cure 20 Mar 4:21 PM (25 days ago)
Two years after Neil Armstrong walked on the Moon, US president Richard Nixon declared a new frontier in American scientific conquest. The target was one of humankind’s biggest killers: cancer. “The same kind of concentrated effort that split the atom, and took man to the Moon, should be turned toward conquering this dread disease,” Nixon declared on December 23, 1971 as he signed the US’s inaugural National Cancer Act. The aim was unequivocal – nothing short of a cure across the gamut of cancers.
Politicians love a lofty goal, but the enemy at the time was barely understood, let alone close to being conquered. Fifty three years on, enormous strides have been made in understanding cancer biology and tumour microenvironments – yet for all of our scientific knowledge, combating the spread of malignant tumours in some of our most deadly cancers is still a losing battle, with the errant cells always one step ahead in their ability to evade both immune fightback and drug weaponry. The fight has been infinitely harder than Nixon imagined.
Cancer is still the second-biggest killer worldwide; three out of every ten deaths in Australia are attributable to it. No other disease touches everyone’s lives in the way cancer does. Almost everyone has a friend or relative who has battled the disease, or in the worst case lost their life to it. Sometimes, it’s the treatment that kills people. Because while the development of immunotherapies and targeted treatments stretches back at least 70 years, the science has been inchingly slow at getting these therapies into the clinic, leaving the blunt tools of chemotherapy and radiotherapy – which often have severe side effects – as the standard treatments for many. So far, monoclonal antibody treatments and other immunotherapies have revolutionised the treatment of only a small suite of cancers.
Yet that may be on the cusp of changing, with a groundbreaking clinical trial proving this year that the majority of melanoma patients whose cancer has spread to the brain can be “cured” when given combination immunotherapy as a first-line treatment. The trial, led by scientists at Melanoma Institute Australia, has presented data that establishes long-term disease control is possible for advanced melanoma patients when given two checkpoint inhibitor immunotherapy drugs, nivolumab and ipilimumab, in combination. The approach is now likely to be replicated in clinical trials across a number of cancers including lung cancer, kidney cancer, head and neck cancer, bladder cancer and triple negative breast cancer.
At the same time, renowned pathologist Professor Richard Scolyer, who was diagnosed with the deadly brain cancer glioblastoma almost two years ago, has been able to extend his life beyond all expectations through a novel personalised “vaccine” that works by activating the immune system and instructing T-cells to kill tumour cells. Scolyer is currently recovering from brain surgery after scans revealed a recurrence in his cancer, but his relative longevity has provided hope for future sufferers as science’s war on cancer occurs across multiple fronts. It’s what federal health minister Mark Butler called a “turbocharged period” of discovery on cancer therapies, after announcing public funding for the first pan-tumour pharmaceutical – Vitrakvi – that has been described as a likely game-changer in precision oncology.
Meanwhile, the combination of technologies such as gene editing has driven advances in new types of immunotherapies, combined with advanced imaging techniques that provide unprecedented insight into immune cells’ responses to cancers. It’s opening up what is set to be the most exciting era yet in effective therapies. “I think we’re looking at a future in which cancer becomes a chronic disease, where we can outlive it,” says Associate Professor Arutha Kulasinghe from the University of Queensland. “I think that’s where we are moving towards. Cancer shouldn’t be a death sentence.”
“The reality is, cancer is so complex. It’s so devious,” says Kerry Strydom, CEO of the Australian Cancer Research Foundation. “It’s not one disease. So the complexity in terms of the treatments – in terms of finding something that even works for a while, and then it stops working as the cancer finds its own way to resist the treatment – is almost overwhelming.
“But innovation is going to cut through all of this. That is what’s going to be able to pull all of this data, all of this research, all of these pieces together, to cut through to an end result. From a technology perspective, this is a moment in time when some of these ideas that researchers have been working on for decades are able to be investigated further. It’s transformational. There is huge potential ahead.”
Few people are watching developments in innovation as closely as Sydney mother-of-two Caitlin Delaney. A scientist by background, she was diagnosed with Stage IV ovarian cancer eight years ago. Her type of cancer – clear cell ovarian cancer – is rare and particularly aggressive. She is only alive today because of her determination to access cutting-edge therapies as a result of her own research. Initially, standard treatment of surgery and chemotherapy was successful, but after two years her cancer recurred. “I was told that standard treatments wouldn’t work for me any more,” Delaney says. “Of course this was very concerning. But that probably did me a favour, because it made me look outside of the hospitals and made me advocate for myself even more. I realised I had to find out who the global experts in my type of ovarian cancer are, what the latest science in the labs is, and how I can access that.”
Delaney had previously pushed for genetic testing, and learned that her tumour had mutations that could be targeted. She then accessed an immunotherapy drug combination off-label, at great expense, that kept her stable for two and a half years. “It made me realise there is hope, I’ve just got to look for the emerging science,” she says.
Since that immunotherapy drug combination stopped working for her, and after more treatments and an aborted clinical trial attempt, her cancer has progressed, leaving her in the position of having to crowdfund further treatment. Now, Delaney is watching the rise of a very promising new form of immunotherapy that re-engineers a patient’s own T-cells, the white blood cells that help the immune system fight off disease. Known as Chimeric Antigen Receptor T-cell therapy, or CAR T-cell therapy for short, it has proven highly effective for blood cancers, but has not yet been able to make the leap to treating solid tumours such as ovarian cancer.
But the eagle-eyed Delaney, who has a degree in biotechnology and who’d signed up to the newsletters and followed market announcements of various biotechs in the cancer space, began to see talk of another type of CAR therapy – this one involving engineered natural killer cells. “I could see there was a lot of money being raised. I had the feeling that this is going be the next thing,” Delaney says. “I just don’t know if I’m going be alive to witness it.”
Inside a small row of bioreactors in a Melbourne laboratory, stem cells derived from umbilical cord blood are forming into three-dimensional groups in a special solution, in a process that mimics the development of one of the most powerful of the body’s immune cells: natural killer cells. NK-cells are lymphocytes of the innate immune system sometimes known as the “tumour killers” that are critical in
detecting and controlling early signs of cancer.
Holding forth in this east Melbourne lab is stem cell biologist Alan Trounson, who decades ago led an Australian team that discovered human embryonic stem cells, and who between 2007 and 2014 headed up the Californian Institute for Regenerative Medicine, a $3 billion stem cell agency that was a world leader in facilitating the translation of stem cell discoveries into clinical therapies. Trounson, an Emeritus Professor Monash University and Distinguished Scientist at the Hudson Institute for Medical Research is also a pioneer of human in vitro fertilisation. There could be few scientists better qualified to utilise the power of stem cell technologies as the basis for engineering an immune cell uniquely primed to conquer one of the deadliest of cancers.
“I’m about research that can actually target something that is a problem,” says Professor Trounson. “I see problems as things that we could possibly solve if we apply the right kind of science to it. We wanted to go after the toughest cancer, the one that hasn’t got a treatment. We chose ovarian cancer. It could have been something else, but ovarian cancer has few therapeutic options. As a scientist, you’re looking for opportunities to make a difference to problems that could be solved using skills and innovation. There’s an opportunity of using stem cells to control ovarian cancer.”
Professor Trounson and his team at the biotechnology company known as Cartherics that he has founded to develop cell immune therapies are engineering natural killer cells that will be super-primed to kill ovarian tumour cells. They’re creating what are known as CAR-NK cells – a type of immune cell engineered to express a chimeric antigen receptor, a type of synthetic receptor for proteins known as antigens that can direct the functioning of immune cells. The CAR-NK cells are specifically designed to target and destroy cancer cells with high efficacy. They contain activating receptors that recognise molecules that are expressed on the surface of cancer cells and virally infected cells which ‘switch on’ the NK cell to kill. They also contain inhibitory receptors that act as a check on NK cell killing. Cancer cells are clever, and often put out signals that activate these inhibitory genes on the NK cell so that it no longer performs its killing function. However, the process of gene editing involved in engineering CAR-NK cells to “knock out” these inhibitory receptors so the immune cells are not blocked from carrying out their function.
“These cells that we make up are even more aggressive at killing tumour cells than the body’s own natural killer cells because they don’t have some of the natural inhibitors that an adult NK cell has, so they just kill dramatically quickly,” Trounson says. “And so we want to deliver these very aggressive natural killer cells directly into the pelvic cavity where the ovarian cancer is. We don’t want to drop them into the blood. We want to put them where the cancer is.”
It is early days for Professor Trounson’s work, but animal studies have provided enough confidence for the US Food and Drug Administration to provide the guidance for an IND to enable a clinical trial which is set to begin in Australia late this year or early next year. There is hope that the emergence of this next wave of cancer immunotherapy – which follows the stunning success in blood cancer of a similar type of therapy known as CAR-T cell therapy – is likely to open up promising treatment pathways for tumours which have long had terrible prognoses. If it proves successful, CAR-NK therapy could have application across a wide spectrum of cancers, including gastric, colorectal, prostate and pancreatic cancers.
At the same time, efforts are underway to extend the application of CAR-T cell therapies to solid tumours. The technology is similar to CAR-NK therapy but rather than being an off-the-shelf product, involves gene editing and re-engineering patients’ own t-cells to fight cancer. It has had success rates of up to 90 per cent in treating blood cancers so far, but has been associated with significant side effects including immune overactivation known as “cytokine storm”, and also neurotoxicity.
The Peter Mac Cancer Centre in Melbourne is at the forefront of adapting CAR-T cell therapies to solid tumours, primarily utilising CRISPR gene editing tools. “There are many advances that are starting to show promise,” says Associate Professor Paul Beavis, immunologist at the Peter MacCallum Cancer Centre. “The field is actually flooded with innovative technologies. So there’s one thousand and one things you can try. The question really is how do people rank them, and which are the ones that are the best to try next?
“My kind of selling point as an immunologist is that because cancers arise from mutations, there’s always the potential for the immune system to recognise these cells as being different. And so as we become more sophisticated in our tools, I think we’ll be able to unlock that.” At the same time as this new wave of cancer immunology research is unfolding, a revolution in understanding of cell biology is developing that has the potential in the future to personalise cancer treatment to a remarkable extent.
Late last year, the Human Cell Atlas project released details of what it described as an unprecedented feat of “human cartography” that outlined cell maps of the human body with a level of detail never before seen. The project has not only described a host of new cell types, but has also revealed exactly how the body’s cells communicate with one another – a mechanism that is particularly important for cancer researchers crafting therapies that harness the body’s own immune response. This kind of three-dimensional digital cell mapping requires advanced imaging techniques and utilises an emerging field of pathology known as spatial biology.
“This will be truly transformative for cancer medicine,” says Kulasinghe. “With traditional approaches, and even when you do genomic testing, you get these sort of bulk profiles of the tissue, but we have no appreciation for where all of the different cells came from in the context of the tissue. So even in CAR-NK or T-cell therapies, there’s no appreciation for what immune cells are actually doing. Now, we have context for every cell within the organ of disease. So we know where the T-cells are. We know where the NK cells are. We know where the tumour cells are. Are they knocking on the door of the cancer cells? Are they at that interface where the tumour cells are interacting with the immune cells? Or are they in the middle of the tumour, for example?
“With these new cell mapping techniques you’ve taken that story of biology and turned it into a digital image of that patient’s tumour. And so we can ask the question, you know, do the NK cells or the T-cells or the macrophages outside the tumour, how do they compare to the immune cells within the tumour? And then you start to learn things like cell patterning. So what are the patterns associated with patients that respond to therapy? What are the patterns associated with patients that don’t respond to therapy? Are the right types of immune cells recognising the tumour? Can we see it? Do we know that it’s engaging all of the tumour? Do we know areas in that ‐ tumour that have no immune cells?
“And so we’re getting more appreciation for where these immune cells are, and the role of those immune cells. Are they exhausted? Are they active? Are they killing the tumour cells? We’re no longer acting off assumptions. We know exactly where these cells are – and that seems to be the transformative piece now in immunology, in tissue mapping, because where these cells are in the tissue is critical, and we’ve never had the tools to be able to look at them before.
“A tumour is always actively trying to mask itself from the immune system, trying to cloak itself. So in the future we’ll have much greater insight into how we can de-cloak these tumour cells, expose immune cells to the tumour and the tumour microenvironment, and then let your body go and kill those [cancerous] cells or turn them off.”
It is likely, as science continues its battle against a constantly evolving enemy, that greater understanding of how to harness the strength of the body’s own immune system will be the most powerful weapon in clinicians’ armoury in treating cancer. Splitting the atom and setting foot on the Moon were astonishing feats of science. Perhaps no one could have predicted combating cancer would turn out to be so much harder.
Article sourced from The Australian website here.
The post The end of cancer: how cell therapy breakthroughs have us on the edge of a cure appeared first on ACRF.
Funding to support continuity of Zero Childhood Cancer for all Australian kids with cancer, and expansion to include young Australian adults with cancer 6 Mar 1:35 PM (last month)
Zero Childhood Cancer (ZERO), a world-leading national precision medicine program for children and young people with cancer, will receive a $112.6 million investment from the Australian Government to extend and expand so that more children and young people can benefit from the latest in scientific and clinical understanding.
Led by Children’s Cancer Institute and Kids Cancer Centre at Sydney Children’s Hospital, Randwick, and involving all of Australia’s children’s hospitals, the funding will ensure ZERO can continue to support this world first delivery of cutting-edge cancer care to all children with cancer (0-18 years), and expand the program to be available to many young people aged 19 to 25 with paediatric type cancers.
When a child is enrolled in ZERO, no matter where they live in Australia, a sample of their cancer and normal tissue are sent to Children’s Cancer Institute and ZERO partner organisations, where scientists and clinicians analyse it at a genomic level so they can identify which treatment and drugs are most likely to be effective.
Following multidisciplinary discussions, the patient’s doctor receives a report that highlights the critical genetic features of the tumour that may influence diagnosis, prognosis and treatment strategies. Where possible, for high risk and complex cancers, those drugs are tested to see how they perform in laboratory models of the individual child’s cancer.
“The impact of childhood cancer is far greater than most people realise,’ explained Professor Michelle Haber AM, Executive Director of Children’s Cancer Institute. “In Australia, we have more than a thousand cases diagnosed every year, and globally, this number is estimated to be well over 400,000. These children endure gruelling treatment with life-long physical, emotional and psychological consequences. For them and their families, life is never the same again.”
“Nowhere else in the world do children with cancer have the opportunity of benefiting from a precision program of this depth and impact,” commented Professor Haber. “ZERO is showing just what’s possible when you combine cutting-edge research and technology with a multidisciplinary team approach to drive clinical care.”
This announcement not only ensures ZERO will continue to be available to all children in Australia with cancer but extends its support to young people aged 19-25 with cancers that are typically seen in childhood, including young people whose childhood cancer has relapsed. The expanded program is expected to support an additional 300 young Australians with cancer each year, totalling approximately 1300 children and young people annually who will have access to ZERO’s comprehensive precision medicine platform.
The Hon Mark Butler MP, Federal Minister for Health and Aged Care, said ZERO was playing a significant role in improving outcomes for children and young people with cancer.
“The ZERO Childhood Cancer program has already supported more than 2,000 Australian children with cancer, and thanks to this investment, the team will be able to expand that support to another 300 young Australians each year.
“The precision medicine that these world-leading programs make possible is a real game-changer in cancer care – particularly for children and adults with rare or otherwise incurable cancers.”
Professor David Ziegler, Senior Specialist at the Kids Cancer Centre, Sydney Children’s Hospital, Randwick, Chair ZERO2 Clinical Trial and Group Leader of the Brain Tumour Group at Children’s Cancer Institute, said ZERO was changing the model of care for children with cancer.
“ZERO’s results clearly demonstrate the power of precision medicine to change clinical outcomes. ZERO’s targeted, personalised approach represents a whole new model of care that has the potential to not only improve survival but also reduce damaging side effects in kids and therefore cut down on the time they need to spend in hospital. Ultimately this will be less disruptive for their families and achieve better results at a lower cost to the healthcare system.”
One child to benefit from ZERO is Carys Dawson, who was diagnosed with acute lymphoblastic leukaemia at six years old. Despite initially responding well to treatment, Carys relapsed two years later. ZERO analysis at the time of relapse showed that she actually had a rare sub-type of leukaemia known as Philadelphia chromosome-like acute lymphoblastic leukaemia. This information was used to change her treatment and today Carys is back at school, having just started year 6.
Laura Dawson, Carys’ mum, says that without the ZERO results the outcome for Carys could have been very different.
“Without the Zero Childhood Cancer Program, my daughter would have continued the pattern of ‘recovery’ and relapse with tragic results. She was being treated for the most common type of leukaemia, and responding well, but it turned out she had a very rare sub-type and needed a different treatment approach. Everyone was shocked by the genomic test result, and it saved her life.”
ZERO is changing the future for children with cancer the world over and with ongoing support, it will continue to lead the world in precision medicine for children with cancer.
“Our goal is to create a future where precision medicine is embedded in our health system as the new model of care for all children and young people with cancer in Australia,” commented Associate Professor Vanessa Tyrrell, Zero Childhood Cancer Program Director. “With funding now secured, we can integrate emerging research to continuously improve the ZERO platform, while also planning how to embed ZERO as a permanent solution for all children and young people impacted by cancer in the future.”
Australian Cancer Research Foundation (ACRF) awarded CCI a $1.5 million grant in 2014 to help establish the ACRF Child Cancer Precision Medicine Centre, which is now the headquarters of this cutting-edge ZERO program that is transforming outcomes for families impacted by cancer.
Article sourced from Children’s Cancer Institute website here.
The post Funding to support continuity of Zero Childhood Cancer for all Australian kids with cancer, and expansion to include young Australian adults with cancer appeared first on ACRF.
60km in May Challenge Now Open: Walk or Run to Support Lifesaving Cancer Research with Australian Cancer Research Foundation (ACRF) 5 Mar 2:27 PM (last month)
Registration Now Open for ACRF’s Annual Fundraising Challenge
Australian Cancer Research Foundation (ACRF) is proud to announce that registration for our popular “60km in May” challenge is now open. It’s a nationwide fitness and fundraising event that invites participants to commit to covering 60 kilometers throughout May while raising vital funds for breakthrough cancer research.
How It Works
The concept is simple:
– Register online at fundraise.acrf.com.au/event/60km-in-may
– Set your personal fundraising goal
– Cover 60km your way – walk, run, jog, or combine activities throughout May
– Share your journey with friends and family to encourage donations
Participants can tackle the challenge solo or form teams with friends, family, or colleagues. The flexible format allows you to complete your kilometers at your own pace, whether that means 2km daily or longer weekend sessions.
Why 60km?
The distance represents the journey of resilience that people impacted by cancer face. By pushing yourself to reach 60km, you’re showing solidarity with those affected by cancer while directly contributing to research that saves lives.
Supporting Breakthrough Research
Every dollar raised through the 60km in May challenge will help fund innovative cancer research projects across Australia. ACRF provides grants for cutting-edge equipment and technologies that enable researchers to accelerate discoveries and develop new treatments.
This year, with your support, we aim to raise $300,000 to help fund lifesaving research projects nationwide.
Who Can Participate?
Anyone can join the challenge, regardless of fitness level or age. The event is designed to be inclusive and accessible:
– Fitness enthusiasts can push their limits
– Beginners can break the distance into manageable daily walks
– Families can participate together, making it a meaningful activity for children
– Workplace teams can use it as a team-building opportunity while supporting a vital cause
Registration Details
Registration is free and comes with access to:
– Personal fundraising page
– Tracking tools to log your progress
– Fundraising tips and resources
– Supportive online community
– Official 60km in May merchandise (the more you fundraise!)
Be among the first 1,000 participants to register, and you’ll receive a FREE ACRF branded sweatband to wear during the challenge.
Join the Movement
With one in two Australians diagnosed with cancer by the age of 85, this devastating cause touches almost every family. By participating in the 60km in May challenge, you’re taking direct action to change the future of cancer treatment and prevention.
Register today and be part of the movement toward a world without cancer.
About ACRF: Australian Cancer Research Foundation funds brilliant cancer research, providing grants for cutting-edge equipment and technology. Since 1984, ACRF has awarded over $204 million in cancer research grants, helping to accelerate the discovery of new ways to prevent, detect and treat cancer.
The post 60km in May Challenge Now Open: Walk or Run to Support Lifesaving Cancer Research with Australian Cancer Research Foundation (ACRF) appeared first on ACRF.
The Old Blokes are back on the road again in 2025, raising funds for Australian Cancer Research Foundation (ACRF) 4 Mar 3:41 PM (last month)

In 2023, the Old Blokes Driving for a Cancer Cure raised a record-breaking $102k for Australian Cancer Research Foundation (ACRF).
This year, they’re aiming to smash their previous target and raise more vital funds for cancer research. You can support the Old Blokes by donating to their fundraising page: https://donate.acrf.com.au/event/oldblokes
The Old Blokes Driving for a Cancer Cure are a group of car enthusiasts bonded by their connection to cancer.
‘Cancer has touched all of our lives in some way, whether through the loss of a loved one, a friend, or a colleague. Personally, the loss of my father, father-in-law, work colleagues, and close friends to this devastating disease has inspired me to take action.’ – Mario Nearchou, OBDCC Lead
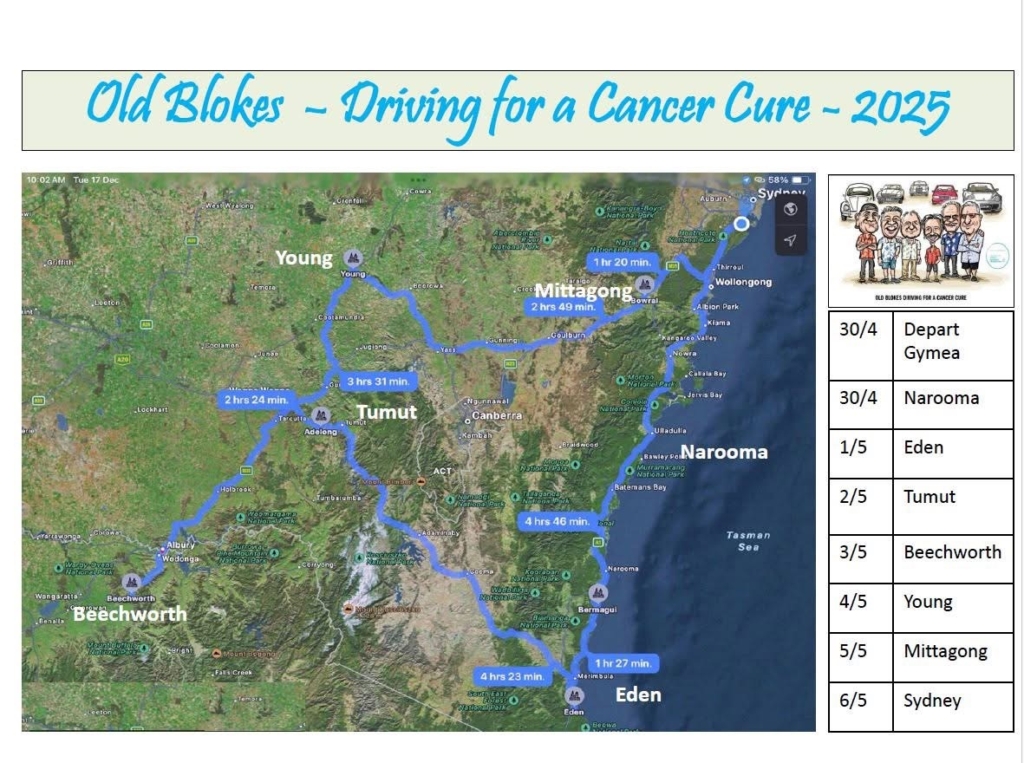
On April 30, the Old Blokes will embark on a seven day journey in their classic cars across NSW and VIC.
The Old Blokes will stop in a few towns along the way, to raise awareness of the work ACRF does and encourage people to support their fundraising efforts.
Please see their route below:
If you would like to come along the drive, the Old Blokes ask you donate $300 to their fundraising page. In return, you will receive an Old Blokes t-shirt and a mug.
The Old Blokes will also host a fundraising dinner on October 17 at Doltone House in Sylvania Waters, NSW. Please get in contact with Mario Nearchou at 0416 207 723 if you are interested in attending.
‘I dream of a world free from cancer, and I’m determined to help make that a reality.’
We cannot wait to see what the Old Blokes achieve in 2025!
Make sure to follow their journey on Instagram @oldblokes
The post The Old Blokes are back on the road again in 2025, raising funds for Australian Cancer Research Foundation (ACRF) appeared first on ACRF.
ONJCRI Researchers Pave the Way for Next-Generation Gene Editing in Cancer Research 2 Mar 2:28 PM (last month)
Researchers at the Olivia Newton-John Cancer Research Institute (ONJCRI) have made an unprecedented advancement in cancer research by using the gene-editing tool Cas12a in a newly-developed pre-clinical model. Importantly, their work led to the identification of genes that drive lymphoma growth, a type of blood cancer. Their findings, published today in Nature Communications, were made possible through a collaboration between researchers at ONJCRI, WEHI and Genentech, a member of the Roche Group.

Dr Yexuan Deng, Dr Eddie La Marca and Wei Jin, all co-lead authors on the paper
What is Gene Editing?
Our bodies consist of trillions of cells, each containing a set of instructions called genes. These genes are written in a special code called DNA, which determines how cells grow and function. When this code has errors, diseases like cancer can develop.
To solve this problem, researchers developed CRISPR, a powerful tool that acts like a molecular “search and edit” function for DNA, and more recently, RNA – the cellular messenger that carriers out DNA’s instructions. The key to this technology is a special protein called a Cas enzyme, which acts like molecular scissors, cutting and modifying specific DNA or RNA sections. By editing faulty genes, researchers aim to find better ways to treat cancer.
How is this Study Advancing Gene Editing?

Until now, most research has relied on the Cas9 enzyme for gene editing. This study is the first to demonstrate Cas12a usage in a pre-clinical cancer model. Cas12a offers distinct advantages, including the ability to edit multiple genes at once with high precision.
Using this tool, our researchers identified genes that accelerate lymphoma growth. They achieved this by using a specially designed set of genetic “libraries” tailored to work with newly established Cas12a-compatible mice. This allowed them to scan the entire genome (the complete instruction manual for an organism) and pinpoint the genes contributing to cancer.
Dr Eddie La Marca, a researcher at ONJCRI and WEHI, explains:
“This is the first time Cas12a has been used in pre-clinical models, and it will greatly enhance our ability to study cancer. Unlike Cas9, Cas12a allows us to delete multiple genes at once with extremely high efficiency.”
By combining Cas12a with other gene-editing tools, the team also created a system that can both delete faulty genes and activate beneficial ones at the same time, a technique known as multiplexed gene editing.
The Future of Gene Editing in Cancer Treatment

Professor Marco Herold, Chief Executive Officer of the ONJCRI and Head of the La Trobe University School of Cancer Medicine, emphasised the significance of this breakthrough:
“We are certain that this work will encourage other research teams to adopt this Cas12a pre-clinical model, which, combined with screening libraries, offers a powerful new set of gene-editing tools to enhance our understanding of the mechanisms behind many different cancers.”
Professor Herold’s team at the ONJCRI is now focused on developing methods to deliver CRISPR-based therapies to patients, bringing gene-editing technology one step closer to being used in novel cancer treatments.
“This Cas12a pre-clinical model will be key in advancing our understanding of how CRISPR tools can be applied in clinical settings.”
This research was made possible with thanks to generous funding from the National Health and Medical Research Council (NHMRC) and Phenomics Australia.
Read the full publication in Nature Communications.
In 2022, Australian Cancer Research Foundation (ACRF) awarded a grant of $2.1 million to establish the ACRF Centre for Precision Medicine at the Olivia Newton-John Cancer Research Institute (ONJCRI). Here scientists will explore theranostics, a form of precision medicine in which radioisotopes are combined to diagnose and treat a tumour.
Original article source: https://www.onjcri.org.au/latest-news/staff/cas12a-nature-communications/
The post ONJCRI Researchers Pave the Way for Next-Generation Gene Editing in Cancer Research appeared first on ACRF.